|
Fundamental Research in Membrane Separations
-
Membrane
Contactor as Degasser: simulation studies
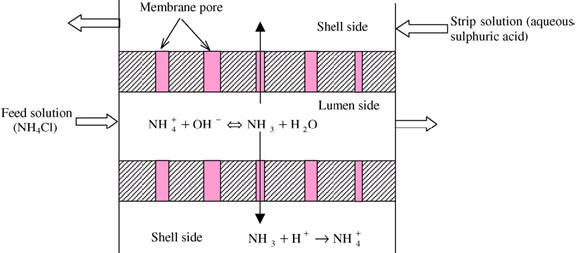
Membrane contactor as degasser
(from
water) can be either
operated under vacuum or under LLE modes. Numerical
simulations of mass transfer under different flow
conditions have been attempted in order help to design
the hollow fibre (hydrophobic polymer) based membrane
contactor. Mathematical models have been developed* by
using the resistances-in-series mass transfer system
that takes into account boundary layers, membrane
porosity, phenomenological considerations and mass
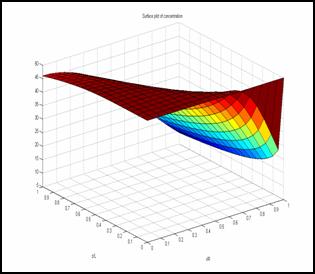
balances of the membrane contactor. Simulation result
shows the variation of concentration in both axial and
radial direction.
*
Amish Mandowara and Prashant K. Bhattacharya, “Membrane
contactor as degasser operated under vacuum for ammonia
removal from water: A numerical simulation of mass
transfer under laminar flow conditions”, Computers
Chem. Eng. (DOI information:
10.1016/j.compchemeng.2008.12.005).
-
Pervaporation:
membrane casting, separations and modelling
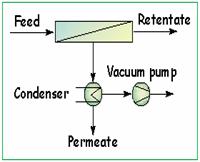
Pervaporation using hydrophobic membranes were observed
to be promising for streams such as water contaminated
with VOC's, organic-organic solutions, etc.
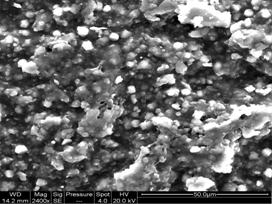
Membrane Casting:
Several polymeric hydrophobic and hydrophilic dense
membranes* were cast, modified and characterized
through FTIR, XRD, 1H NMR, SEM, AFM, etc. Specific systems like
hydrazine-water, alcohol-water, organic-organic, etc.
have been studied. They were also assessed in comparison
to commercial membranes for several typical chosen
mixture separations.



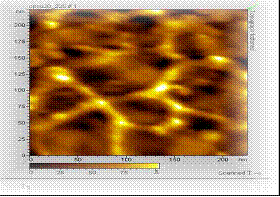 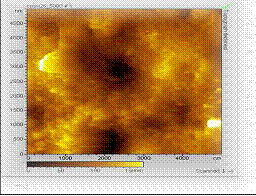
*
S. V. Satyanarayana, A. Sharma and P. K. Bhattacharya,
“Composite membranes for hydrophobic pervaporation:
Study with toluene-water system“, Chem. Eng. J., 102,
171 -184 (2004).
* S. V. Satyanarayana, P. K. Bhattacharya,
“Pervaporation of Hydrazine Hydrate: Separation
characteristics of membranes with hydrophilic to
hydrophobic behaviour“, J. Membrane Sci., 238,
103 -115 (2004).
* Mrinal K. Mandal and P. K. Bhattacharya, "Poly
(ether-block-amide) membrane for pervaporative
separation of pyridine present in low concentration in
aqueous solution", J. Membrane Sci., 286, 115-124
(2006)
* Mrinal K. Mandal and P. K. Bhattacharya, “Poly (vinyl
acetal) membrane for pervaporation of benzene-isooctane
solution”, Separ. Purif. Technol., 2007, 61
(2008) 332–340.
Real coded genetic algorithm and
Model Development:
Models have been developed to predict partial fluxes
and selectivity's'. Real coded genetic algorithm has
been used to optimize process parameters.
* Gopal R. Nemmani, Satyanarayana V. Suggala and
Prashant K. Bhattacharya, “NSGA-II for Multiobjective
Optimization of Pervaporation Process: Removal of
Volatile organics from Water”, Ind. Eng. Chem. Res.
(in press).
*
Satyanarayana V. Suggala and Prashant K. Bhattacharya,
“Real Coded Genetic Algorithm for Optimization of
Pervaporation Process Parameters for Removal of Volatile
Organics from Water“, Ind. Eng. Chem. Res., 42 (13),
3118 -3128 (2003)
* Nazish Hoda, Satyanarayana V. Suggala and Prashant K.
Bhattacharya, “Pervaporation of Hydrazine – Water
through Hollow Fiber Module: Modeling and Simulation“,
Computers Chem. Eng., 30 (2), 202-214 (2005).
Roles of Permeant-Membrane Interactions:
The membrane permeation of a component followed by its
vaporization on the opposite face is governed by the
solubility and downstream pressure. We studied and
measured* the evaporative flux using dense membranes
with different free volumes and different affinities (wettabilities
and solubilities) for the permeant. Interestingly, the
evaporative flux for different membranes vanished
substantially (10-75%) below the equilibrium vapour
pressure in the bulk. The discrepancy was larger for a
smaller pore size and for more wettable membranes
(higher positive spreading coefficients). This
observation, which cannot be explained by the existing
(mostly solution diffusion type) models of pervaporation,
suggests an important role for the membrane-permeant
interactions in nanopores that can lower the equilibrium
vapor pressure.
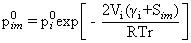
*
A. Sharma, S. P. Thampi, S. V. Suggala and P. K.
Bhattacharya, Pervaporation from a dense membrane: Roles
of permeant – membrane interactions, Kelvin effect, and
membrane swelling, Langmuir, 20(2004), 4708.
Location of vaporization using single component
pervaporation:
A mathematical model* was developed assuming two zones
inside the membrane; namely, liquid permeation and vapour permeation zones. Considering a pressure
distribution across the thickness of the membrane, Kelvin
equation (saturation vapour pressure gets
modified inside the membrane due to permeant membrane
interactions) proved to be useful in developing the
model.
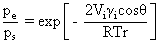
* Sumesh P. T. and P. K. Bhattacharya, “Analysis of
Phase Change during Pervaporation with Single Component
Permeation", Colloids and Surfaces A: Physicochemical
and Engineering Aspects, 290, 263–272 (2006).
Positron annihilation study of pervaporation dense
membranes*:
Determination of free volume sizes is crucial to the
understanding of pervaporation process. Positron
annihilation technique has been developed into a
powerful characterization tool for the study of free
volume and free volume fraction in polymers. Long-lived
components (lifetimes in the range 1.4 to 3 ns) were
found, which were attributed to ortho-positronium (o-Ps)
pick-off annihilations in free volumes. Free volume data
is used to interpret the data of hydrazine hydrate
separation by pervaporation.
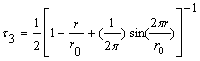
*
S. V. Satyanarayana, V. S. Subrahmanyam, H. C. Verma, A.
Sharma, P. K. Bhattacharya, “Application of positron
annihilation: Study of pervaporation dense membranes“,
Polymer, 47, 1300-1307 (2006).
-
Development
of Predictive Models for Flux Decline
Osmotic pressure controlled (for low MW solutes) and
gel-layer controlled (for high MW solutes) flux decline
have been examined by several workers with regard to
modelling of UF flux behaviour. The applicability of
either of these models is questionable for all operating
conditions and solutes, especially in the intermediate
range of molecular weights. Several predictive models
have been developed overcoming these limitations.
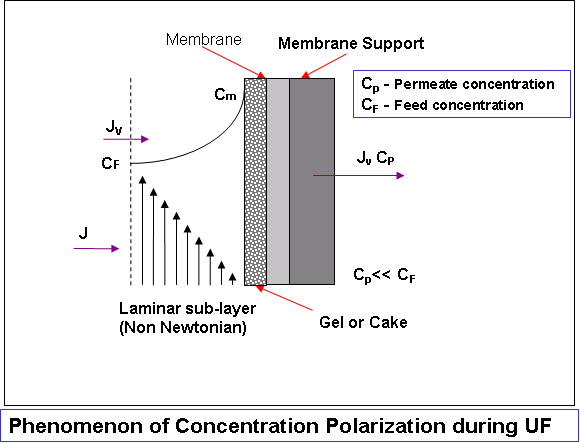
The combined influence of osmotic pressure and gel-layer
on the flux decline is to be expected during UF with
solutes that display substantial osmotic pressure, and
in addition, either form a true gel or a 'pseudo gel'.
Such excellent models* can be useful for design purposes
of UF and RO.

* C. Bhattacharjee and P. K. Bhattacharya, “Prediction
of limiting flux in ultrafiltration of kraft black
liquor“, J. Membrane Sci., 72, 137-147 (1992).
* S. Ganguly and P. K. Bhattacharya, “Development of
concentration profile and prediction of flux for
ultrafiltration in a radial cross flow cell“, J.
Membrane Sci., 97, 185-198 (1994).
* S. De and P. K. Bhattacharya, “Flux prediction of
black liquor in cross-flow ultrafiltration using low and
high rejecting membranes“, J. Membrane Sci., 109,
109-123 (1996).
* S. Bhattacharjee, A. Sharma and P. K. Bhattacharya, “A
unified model for flux prediction during batch cell
ultrafiltration“,
J.
J. Membrane Sci,
111, 243-258 (1996).
-
Role
of Surface Interactions
The approach is to systematically incorporate the
intermolecular interactions between solutes and
membranes to develop models of flux decline. The role of
solute-solute interactions is manifested in the
variation of diffusivity and osmotic pressure, which
govern the transport of the solution across the
polarized layer and through the membrane. The
solute-membrane interactions further affect the
transport of solute and solvent through the membrane
pores, thus predicting adsorption, fouling, etc. Models,
thus, represent a marriage between the surface science
and membrane separations aspects, and provide excellent
agreement with experimental fluxes.
* S. Bhattacharjee, A. Sharma and P. K. Bhattacharya,
“Surface interactions in osmotic pressure controlled
flux decline during ultrafiltration“, Langmuir, 10,
4710-4720 (1994).
* S. Bhattacharjee, A. Sharma and P. K. Bhattacharya,
“Estimation and influence of long range solute-membrane
interactions in ultrafiltration“, Ind. Eng. Chem.
Res., 35(9), 3108-3121 (1996). [Invited paper for the special issue in honour of Prof.
E. Ruckenstein].
-
Mass Transfer Coefficient with Suction
Sherwood number relations for the prediction of mass
transfer coefficient, including the effect of suction,
have been developed from first principles]. The proposed
relations predict the permeate flux in RO and UF in an
excellent manner. For other effluents, some empirical
equations have also been developed.
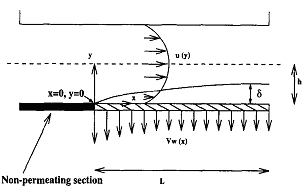
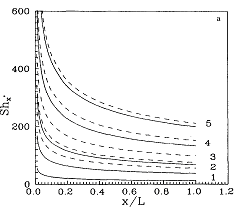
* S. De and P. K. Bhattacharya, “Mass transfer
coefficient with suction including property variations
in applications of cross-flow ultrafiltration“, Separ.Purif. Technol., 16, 61-73 (1999).
* S. De and P. K. Bhattacharya, “Prediction of mass
transfer coefficient with suction in the applications of
reverse osmosis and ultrafiltration“, J. Membrane Sci.,
128, 119-131 (1997).
-
Generalized Integral & Similarity
Solutions for Concentration Profiles
In this exciting method, one has to solve only two
ordinary differential equations and one non-linear
algebraic equation, compared to solving around 700
coupled ordinary differential equations simultaneously
in the detailed numerical solution. Still accuracy and
generality of the problem are fully retained. The model
can predict flux decline over time (for an unstirred) or
over channel length (for a steady cross-flow system) as
well as of the permeate concentration with time or
channel length, if operating conditions are known.
* S. De, S. Bhattacharjee, A. Sharma and P. K.
Bhattacharya, “Generalized integral and similarity
solutions for concentration profiles for osmotic
pressure controlled ultrafiltration“, J. Membrane,
Sci., 130, 99-121 (1997).
Back |